Diagnosis, Monitoring and Prediction of BCG Therapy Response for Bladder Cancer
Oncuria® is the first-of-its-kind multiplex protein-based immunoassay that detects 10 biomarkers consistent with bladder cancer.
CLIA-Certified Lab Service
Research Service
Early Detection
Diagnosis
Therapy Selection
Therapy Monitoring
Introducing Oncuria® Test
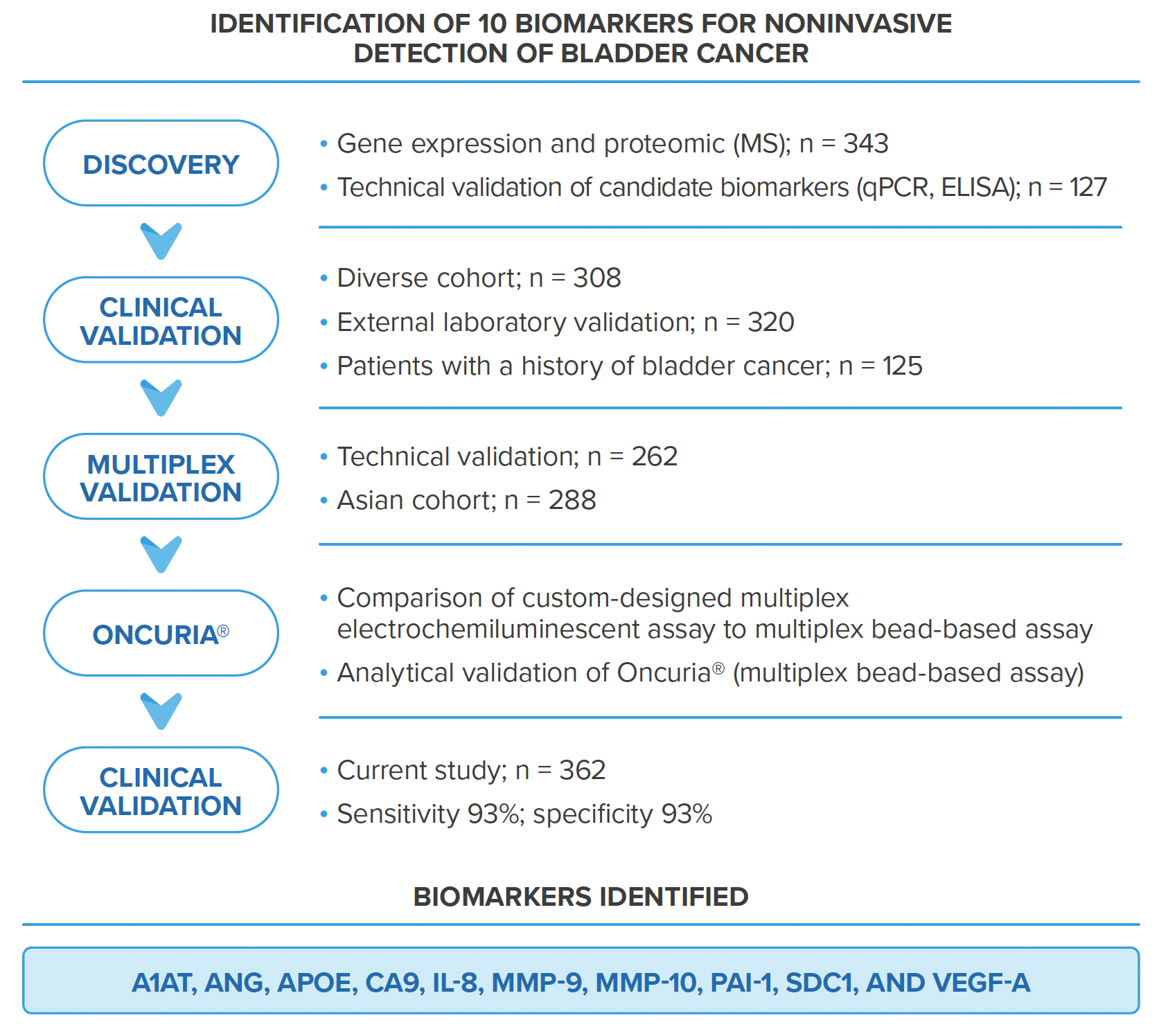
Oncuria® is designed to quantify 10 protein biomarkers, aiming at a bladder cancer associated diagnostic signature in voided urine samples from individuals who are suspected of bladder cancer. It includes three tests: Oncuria® Detect, Oncuria® Monitor, and Oncuria® Predict.
Home collection kit is available for convenience and ease of use.
Urine sample
Oncuria® has a sensitivity of 93% and specificity of 93% for diagnosis
Oncuria® has a 85% negative predictive value for surveillance
Oncuria® has a 90% sensitivity when deciding on BCG treatment for bladder cancer

Oncuria® can detect biomarkers consistent with bladder cancer in patients with gross and micro-hematuria.
The primary diagnostic tool for bladder cancer is cystoscopy and urine cytology (VUC). However, cystoscopy uses an invasive procedure with a risk of causing infection and trauma. Although VUC is often chosen for the noninvasive detection of bladder cancer, it gives suboptimal sensitivity despite high specificity.
In recent years, multiple tests have been developed to diagnose bladder cancer. Still, the sensitivity or specificity is either low or varies widely. A consistent high-performance non-invasive bladder cancer diagnosis test is highly desired.
Oncuria® test is exactly such a test for bladder cancer diagnosis!
Bladder Cancer
Sixth
Most incident cancer
Ninth
Most deadly cancer

Oncuria® helps monitor people with early-stage bladder cancer for recurrence.
Bladder cancer often recurs even among the patients who get appropriate treatment. Bladder cancer recurrence occurs in non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) types. It is hard to know when the cancer will come back. Therefore, adequately scheduled surveillance is necessary following cancer treatment.
Standard surveillance care includes repeated cystoscopy and cytology. The frequencies of these procedures have been recommended in multiple guidelines based on the patient’s risk level. However, these surveillance tools can take expensive healthcare resources.
Current biomarker assays may effectively monitor bladder cancer recurrence and allow cystoscopy and cytology scheduling to be more logical and targeted, focusing mainly on higher-risk groups.
Oncuria® bladder cancer test monitors the NMIBC patients for cancer recurrence with great sensitivity and specificity.
over
%
Bladder Cancer Patients Experience Cancer Recurrence

Oncuria® can help identify people who are more likely to experience rapid failure with BCG therapy
Bacillus Calmette-Guerin (BCG), a live attenuated tuberculosis vaccine that acts as a non-specific immune system stimulant, has proven to be the most successful adjuvant treatment for non-muscle invasive bladder cancer (NMIBC). BCG helps eradicate residual disease, reduce recurrence, and decrease cancer progression.
Due to the global BCG shortage, predicting which patients respond to BCG therapy and which patients are resistant would be beneficial. Thus, BCG can be effectively allocated to patients who respond to the therapy. However, currently, there are no accurate evaluation tools to predict patient response to intravesical BCG.
Oncuria® test shows high sensitivity and specificity in predicting response to BCG therapy. The test benefits both patients and the healthcare systems to better tailor treatment regimens for individual patients.
Among patients treated with BCG
%
Have Recurrence
%
Have Disease Progression
Disclaimer: This laboratory developed test (LDT) is performed at DiaCarta Laboratory and is designed to test certainproteins known to assist in determining the risk score of bladder cancer from urinary specimens. NonagenBioscience is currently seeking U.S. Food and Drug Administration (FDA) approval for Oncuria as an in vitrodiagnostic test. For questions about this test or to speak with a DiaCarta Support Specialist, please emailsupport@diacarta.com or call (800) 246-8878.
The DiaCarta offerings
SERVICE
Oncuria® is available at DiaCarta CLIA as a Lab-Developed-Test (LDT).
- Acceptable specimens: 2 tubes of urine sample per patient (minimum 5mL). Patients must have serum creatinine </= 2.0 mg/dl and more than 7 days after an invasive procedure.
- Method: multiplex bead immunoassay
- Oncuria® Detect: CPT code 0365U, service catalog number DC-03-1004
- Oncuria® Monitor: CPT code 0366U, service catalog number DC-03-1005
- Oncuria® Predict: CPT code 0367U, service catalog number DC-03-1006
Resources
Disclaimer: This laboratory developed test (LDT) is performed at DiaCarta Laboratory and is designed to test certain proteins known to assist in determining the risk score of bladder cancer from urinary specimens. Nonagen Bioscience is currently seeking U.S. Food and Drug Administration (FDA) approval for Oncuria as an in vitro diagnostic test. For questions about this test or to speak with a DiaCarta Support Specialist, please email support@diacarta.com or call (800) 246-8878.
