Prostate Test
A New Tool To Improve Risk Stratification In Localized Prostate Cancer
CLIA-Certified Lab Service
What is OncoAssure Prostate?
The diagram shows the two intended uses of the tests in the context of prostate cancer diagnosis and management.
OncoAssure is used to estimate the risk of having aggressive disease (post-biopsy) or the risk of biochemical recurrence (post-surgery).
OncoAssure incorporates the well-known and validated clinical assessment tool, Cancer of the Prostate Risk Assessment (CAPRA), into the risk score calculation.

OncoAssure employs a novel approach by utilizing master driver genes, which cover a wide range of biological pathways. The prognostic signature includes 4 prognostic genes (FOXM1, MCM3, MTUS1, TTC21B) and 2 reference genes (ALAS1, PPP2CA).
OncoAssure provides prognostic information regarding contemporary clinical features associated with tumor aggressiveness and poorer clinical outcomes, including cribriform morphology and Tertiary Grade 5
Clinical significance of the Oncoassure Prostate Test
OncoAssure Prostate is a newly developed laboratory test (LDT) designed to help stratify risk in prostate cancer patients. This test is based on data from over 1,000 prostate cancer patients and has demonstrated performance comparable to or better than existing market alternatives.
The OncoAssure Prostate test is a multigene prognostic signature incorporating clinicopathological information which assesses the probability of aggressive disease in men recently diagnosed with early-stage prostate cancer or men with localized disease who have undergone radical prostatectomy (RP).
Men whose OncoAssure Prostate risk score indicates they have a lower risk of aggressive disease may be suitable candidates for active surveillance. After RP, patients with a lower risk of biochemical recurrence can be reassured that surgery is sufficient to control their prostate cancer, whereas patients with a higher risk of recurrence may need adjuvant treatments.
Adverse Pathology Possibility
Patients with a higher OncoAssure Prostate result score were 9.7 times more likely to experience adverse pathology compared to those with a lower OncoAssure Prostate result.
Risk of Biochemical Recurrence
The risk of biochemical recurrence within 5 years was 28.2% for patients with a higher OncoAssure Prostate result, compared to just 6.4% for patients with a lower OncoAssure Prostate result.
%
Reduced Unnecessary Interventions
Using the OncoAssure Prostate test to guide treatment decisions, rather than treating all patients uniformly, could reduce unnecessary interventions by 20–29%.
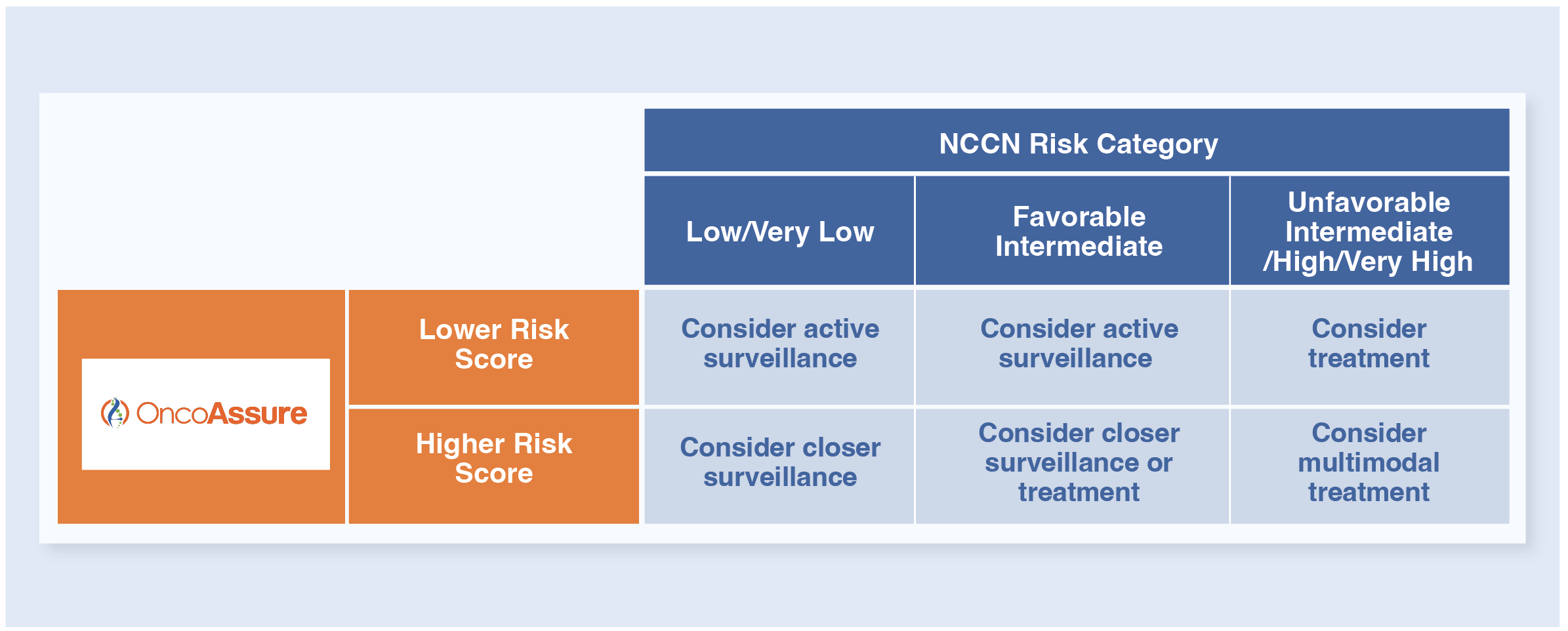
How to Use the OncoAssure Prostate Test?
Based on the OncoAssure Prostate result score on the report (see the interpretation example below), physicians can make recommendations on the treatment strategies (see the table below).
Example Result for a Higher Risk Score Sample

Order OncoAssure Prostate Test
OncoAssure is available at DiaCarta Clinical Laboratory as a Lab-Developed-Test (LDT).
Contact DiaCarta Lab at support@diacarta.com to set up an account
- Download the account setup form.
Send FFPE samples to the DiaCarta lab with complimentary FedEx overnight shipping label.
- Download the Sample Requirement and Shipping Instruction.
- Download the Test Requisition Form.
Review your test results through our secure online portal or receive them via encrypted email.
- Turnaround time: 2 weeks
What payment options are available?
We accept insurance billing as well as direct cash payments. Coverage may vary based on your provider.
Is the OncoAssure test covered by insurance?
Yes, we bill your insurance to cover the basic cost of the test. Coverage depends on your provider and plan. If insurance does not cover it, we offer a cash payment option.
Is financial assistance available?
Yes! Please contact us at support@diacarta.com to discuss available options if you have concerns about payment or insurance.
What happens after my doctor orders the test?
Your doctor’s office will arrange the sample collection and manage the shipping for you. Once the test is complete, the results will be delivered directly to your doctor.
Resources
Disclaimer: The OncoAssure™ Prostate test, a laboratory-developed test (LDT), is performed at our CLIA-certified and CAP-accredited laboratory in Pleasanton, California. It provides clinical value post-biopsy and post-surgery by offering prognostic information regarding adverse pathology and biochemical recurrence, key indicators of metastatic risk, and long-term outcomes. The test has been analytically validated by the laboratory but is not cleared or approved by the U.S. Food and Drug Administration (FDA). For further details or to speak with a support specialist, please contact DiaCarta Clinical Laboratory at support@diacarta.com.”
