ColoScape™ Colorectal Cancer Detection Test
Improved sensitivity for colorectal cancer detection with liquid biopsy
ColoScape™ Colorectal Cancer Detection Test is a novel highly sensitive in vitro diagnostic assay using the qPCR-based multi-gene panel for the qualitative detection of colorectal cancer-associated gene mutations in liquid biopsy and FFPE tissue samples. The kit utilizes our XNA technology which leverages a sequence-specific clamp made by xeno-nucleic acid (XNA) to suppress PCR amplification of wild-type DNA template and selectively amplify only mutant DNA template, reaching sensitivity at 0.1% to 0.5% Variant Allele Frequency (VAF) with 10 ng DNA input. The detection kit identifies the presence or absence of mutations in the targeted regions of four colon cancer-associated genes.
The assay can be performed on qPCR instrumentation that is already available in hospital pathology laboratories. Unlike other colorectal cancer tests on the market, ColoScape™ provides a comprehensive profile of the key colorectal cancer ‘driver’ and ‘resistance’ mutations (gene variation landscape) and offers oncologists valuable information to evaluate targeted therapy options. ColoScape™ could also be used to complement existing colorectal cancer detection tests.
Product Catalog
CE Version: DC-30-0024E
Research-Use Version: DC-30-0024R
Pack Size: 24 Reactions
Service Offering
We provide Lab Testing Services for ColoScape™ Colorectal Cancer Detection Test.
GET A QUOTE NOW
Advantages of ColoScape™ Colorectal Cancer Mutation Detection Test

NON-INVASIVE TESTING
A blood or FFPE sample test intended to detect colorectal cancer mutation at the treatable stage

ultra-sensitivity
Reliably detects 0.1% to 0.5% VAF mutant DNA out of wild-type DNA for targeted mutations

Clinical Sensitivity
66.6% for advanced adenomas (pre-cancer, stage 0); ≥ 88.9% for colorectal cancer (stage I to IV)
low input dna
Minimum 10 ng input DNA per reaction

Diagnostic Aid
Aids colonoscopists in the diagnosis of serrated advanced adenomas
Prediction of Therapy Response
Proprietary XNA technology accurately identifies wild-type and mutant status of relevant genes
Fast Result
Less than 4 hours of assay run time
Comprehensive Coverage
Patented gene panel covering 4 genes and 15 mutations
Great Versatility
Only requires routine qPCR instruments that are available in common research discovery and pathology labs
What Is Colorectal Cancer?
Colorectal cancer is cancer that starts in the colon or the rectum. These cancers can also be named colon cancer or rectal cancer, depending on where they start. Colon cancer and rectal cancer are often grouped together because they have many features in common. Colorectal cancer is the third most common cancer in the world and in the U.S.
Source: American Cancer Society
People died of colorectal cancer in 2018
New cases a year for rectal cancer
New cases a year for colon cancer
New cases a year for colorectal cancer
%
Stage I colon cancer 5-year survival rate
%
Stage I rectal cancer 5-year survival rate
%
Stage IV colon cancer 5-year survival rate
%
Stage IV rectal cancer 5-year survival rate
Death from Colorectal Cancer is Preventable
The trend for the death rate of colorectal cancer has dropped steadily over the years. The development of colorectal cancer takes about 15 years and the death from colorectal cancer is preventable with early diagnosis. Therefore, early screening and diagnosis of colorectal cancer screening is strongly suggested.
According to the American Cancer Society, the guideline for colorectal screening is to start the screening at age 50 (most recent changes to age 45). However, among the 98 million Americans aged 50 to 84, one-third of them are not screened for colorectal cancer for different reasons.
Source: American Cancer Society
The Challenges and Drawbacks for the Current Colorectal Cancer Detection Methods
As a gold standard for colon cancer screening, invasive colonoscopy not only identifies the polyps on the surface of the colon but removes them as well. However, part of the reasons that one-third of the population aged 50 to 84 is not using this screening tool is due to either the unpleasant experience for the preparation of colonoscopy or worries of medical complications caused by bleeding and infection during the process of colonoscopy.
The traditional non-invasive assays such as FIT assays are based on a blood test using immunochemistry, but their sensitivity is much lower (and are therefore inaccurate) compared to the invasive tests.
Molecular diagnostics has only recently been approved for colorectal screening as a powerful non-invasive testing. The currently available ColoGuard test uses stool samples making the screening less inconvenient. However, the stool samples are shipped to a central laboratory for processing and testing, restricting the test availability in local clinical testing labs where patients often seek for these tests. In addition, the test also uses multiple instruments such as liquid handling instruments, qPCR instruments and ELISA readers, making the costly investment a barrier for localization of the test.
Source:
Zauber, et al., Agency for Healthcare Research and Quality (2009)
Pickhardt et. al., Radiology (2011)
Pickhardt et. al.
Johnson, et. al., New England Journal of Medicine (2008)
RadiologyInfo for Patients
Colonoscopy (Invasive)
Colonoscopy is the gold standard for colorectal cancer screening. It allows a doctor to closely to see the inside of the entire colon and rectum for polyps, which could be an early sign of cancer and grow over time to develop cancer.
Sensitivity – Colorectal Cancer: 95%
Sensitivity – Advanced Adenomas: 95%
Specificity: 90%
Sigmoidoscopy (Invasive)
Examination of sigmoid colon (most distant part of
Sensitivity – Colorectal Cancer: ~50%
Sensitivity – Advanced Adenomas: ~50%
Specificity: 92%
CT Colonography (Invasive)
Computed tomography (CT) colonography or virtual colonoscopy uses special x-ray equipment to examine the large intestine for cancer and growths called polyps.
Sensitivity – Colorectal Cancer: 96%
Sensitivity – Advanced Adenomas: 94%
Specificity: 86% to 96%
gFOBT (Non-Invasive)
gFOBT (guaiac
Sensitivity – Colorectal Cancer: Hemoccult SENSA: 70%; Hemoccult II: 40%
Sensitivity – Advanced Adenomas: Hemoccult SENSA: 24; Hemoccult II: 12%
Specificity: Hemoccult SENSA:95%; Hemoccut II: 98%
FIT (Non-Invasive)
FIT (
Sensitivity – Colorectal Cancer: 70%
Sensitivity – Advanced Adenomas: 22%
Specificity: 95%
ColoGuard® (Non-Invasive)
ColoGuard® is an FDA-approved test (2014) for colorectal cancer by checking gene mutations and methylations from stool DNA. Source: ColoGuard®
Sensitivity – Colorectal Cancer: 92%
Sensitivity – Advanced Adenomas: 69%
Specificity: 89%
ColoScape™ (Non-Invasive, suitable for both liquid biopsy and FFPE samples)
ColoScape™ is a novel multi-gene mutationg gene panel qPCR-based assay for the qualitative detection of colorectal cancer (CRC)-associated somatic mutations in the genes that are frequently mutated in colon cancer subjects and that are responsible for aberrant colonic epithelial cell proliferation.
Sensitivity – Colorectal Cancer (Stage I – IV): ≥ 88.9%
Sensitivity – Advanced Adenomas (Pre-Cancer): 66.6%
Specificity (FFPE): ≥ 96%
ColoScape™ is Optimal for Liquid Biopsy
Recently, with liquid biopsy gaining more traction, circulating cell-free DNA in blood samples can be used for testing gene mutations for powerful cancer diagnostics tools. ColoScape™ colorectal cancer mutation detection kit is developed and used after FIT-positive test and before the colonoscopy examination. Preliminary testing data shows ColoScape™ colorectal cancer mutation detection kit has 95.5% sensitivity and specificity of >96% for stage I to IV colorectal cancer and >60% sensitivity for pre-cancer for FFPE samples.
Patented Colorectal Cancer Detection Panel
Based on the colorectal cancer gene mutation panels licensed from the University of Potsdam, 20 mutations in four genes associated with colorectal cancers, APC, BRAF, CTNNB1, and KRAS, are detected in three multiplex qPCR reactions. With the negative, positive, and reference gene controls, the qPCR-amplified mutation target can be used to call out positive or negative results based on the difference in Cq value between the Cq for mutation target and the Cq for reference gene control.
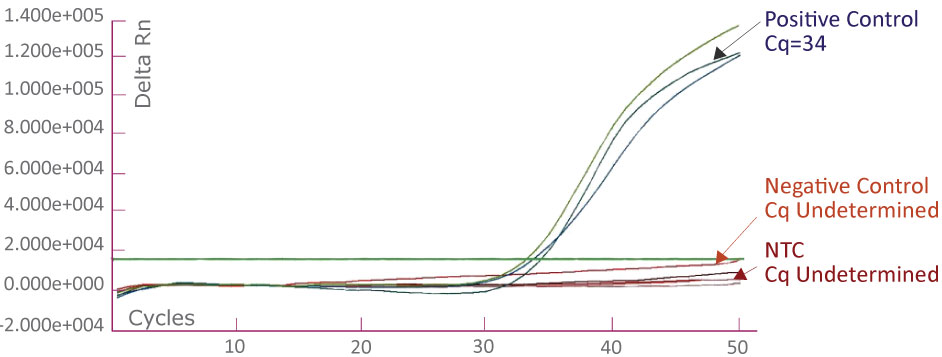
The APC 1367 Amplification Plot on ABI 7500 Fast Dx showing positive control, negative control and no template control
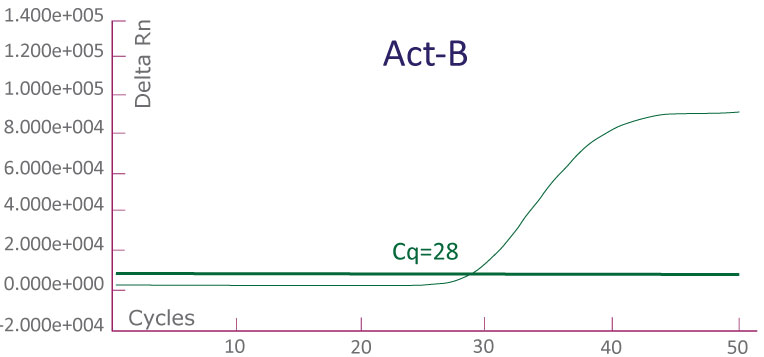
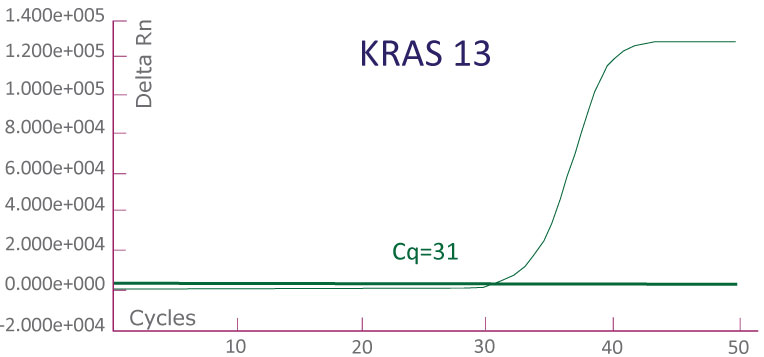
A run file on ABI 7500 Fast Dx showing a KRAS-positive clinical sample
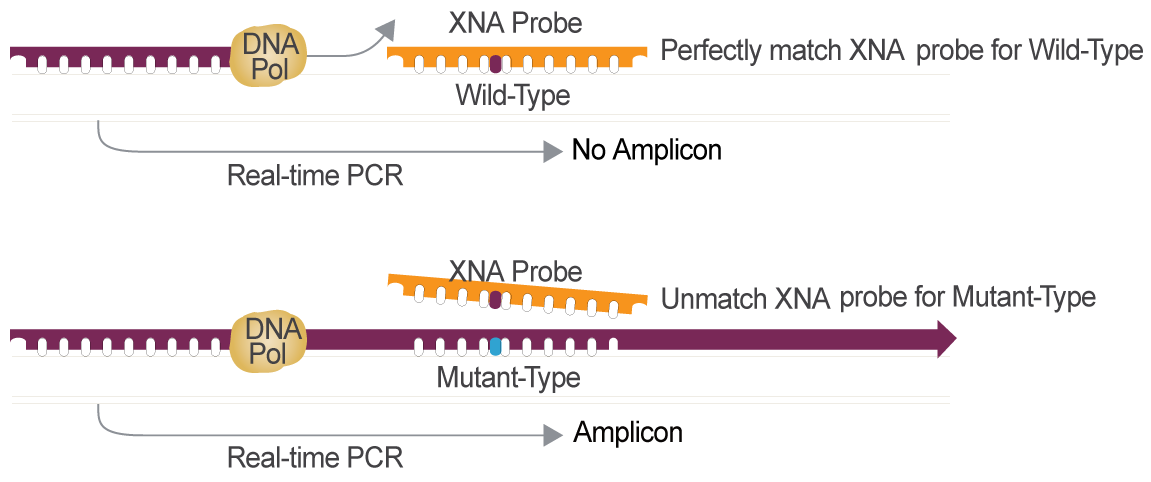
Colorectal Cancer Gene Mutation Detection Powered by XNA Technology
XNA is the Optimal Choice for Cancer Gene Mutation Detection Compared to other Technologies
XNA,

Sanger Sequencing
Advantage: accurate result and is, therefore, the gold standard
Disadvantage: low sensitivity (20% to 25% VAF)

Pyrosequencing Assays
Advantage: better sensitivity and throughput than Sanger sequencing; the early form of NGS assays
Disadvantage: low sensitivity (5% to 8% VAF)

NGS Sequencing
Advantage: high-throughput and good sensitivity (1% to 5% VAF, or even better)
Disadvantage: costly and time-consuming (7 to 10 days)

Digital Droplet PCR (ddPCR)
Advantage: high sensitivity (claimed to be 0.001% VAF)
Disadvantage: much less sensitivity observed in testing than claimed and suffers from false-positive results

qPCR Analysis
Advantage: Sensitivity can reach 1% VAF for some targets. Rapid with minimal hands-on work.
Disadvantage: Multiple methods available for qPCR and a lot of variations in sensitivity. Some of them are only 10% VAF
Streamlined Workflow for ColoScape™ Colorectal Cancer Mutation Detection Test

Step 1: DNA Isolation & Quantification
Extract DNA from FFPE or plasma using a commercial DNA extraction kit and measure the concentration using fluorometric analysis

Step 2: set up qpcr
Mix the assay reagents, load into PCR plate, add controls and extracted DNA ~ 30-60 minutes
Step 3: Amplification parameters
Enter amplification parameters on
qPCR instrument, load PCR plate
and start the run ~ 2.5 hours
Step 4: Data analysis
Determine the presence or absence
of mutations according to the Cq
value cutoffs ~ 15 minutes
Product Specifications for ColoScape™ Colorectal Cancer Mutation Detection Test
Product Catalog Number
CE Catalog Number: DC-30-0024E;
Research-Use-Only (RUO) Catalog Number: DC-30-0024R
Sample Type
FFPE and Plasma
Input DNA
10ng/Reaction
Pack Size
24 Reactions
Instruments Validated
Roche LightCycler® 480, Bio-Rad CFX384. ABI QuantStudio 5 and ABI 7500 Fast Dx
Detection Chemistry
TaqMan
Turnaround Time
Less Than 4 hours
Stability
Stable for 12 months at room temperature (15°C to 25°C)
Webinar
Title: Colorectal Cancer Genetics & A Non-Invasive Test for Colorectal Cancer Mutation Detection Powered by XNA
Speaker: Sir Walter Bodmer (Head of Laboratory for Cancer and Immunogenetics, Weatherall Institute for Molecular Medicine, University of Oxford) and Michael J. Powell,
Highlight:
- Colorectal cancer genetics
- Driver mutations in a limited number of genes are the ideal candidates for screening & early detection of recurrence
- ColoScape™: Discuss future use of early identification of subclinical disease, cancer recurrence & monitoring
Resources
View recent press releases for the ColoScape™ assay: