FDA EUA SARS-CoV-2 qPCR Test Kit
Accurately detects Orf1ab gene with high throughput.
CE/IVD Marked Product
Research Service
The QuantiVirus™ SARS-CoV-2 Multiplex Test Kit is based on Real-Time PCR technology, developed for specific detection of SARS-CoV-2 (COVID-19) viral RNA extracted from nasopharyngeal swabs, oropharyngeal swabs and sputum. The analytical sensitivity is 50 copies per mL of SARS-CoV-2 viral with a 95% confidence.
Looking for a RT-PCR test to detect more genes?
Looking for a testing service? Our CLIA lab is also available for the COVID-19 testing! Learn more >
Product Highlight
- FDA Emergency Use Authorization (EUA) Authorized (submission#: 200575)
- CE/IVD Marked
- Detected gene: Orf1ab
- High throughput: 93 samples (96-well plate) or 381 samples (384-well plate) per run
- Turnaround time: Less than 2 hours from RNA extraction to results
Sensitivity
- Analytical sensitivity: 50 copies per mL of viral RNA
- Clinical sensitivity: 100% agreement with real patient samples previously tested with another assay
Validated Machines
Thermo Fisher (ABI) QuantStudio 5, Thermo Fisher (ABI) 7500 Fast Dx, and Bio-Rad CFX 384
COVID-19 Hotline
Email: covid19support@diacarta.com
Phone #: +1 510-878-6662, option 4 (tech support)
GET A QUOTE NOW
How Does the QuantiVirus™ SARS-CoV-2 Multiplex Test Kit Work?
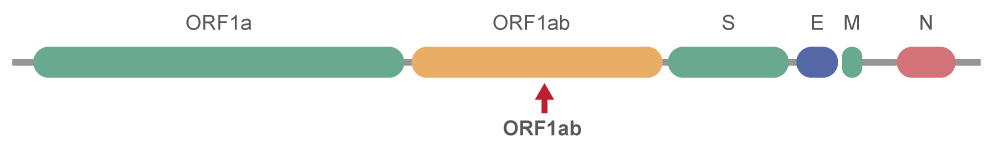
The assay is a multiplex rRT-PCR assay consisting of one reaction with primers and probes for the viral targets (Orf1ab gene) and internal control in one tube thus with increased assay throughput and ease of use and other advantages as a multiplex assay.
The Orf1ab gene of the SARS-CoV-2 genome is targeted in the rRT-PCR assay. Primers and TaqMan probes designed for conserved regions of the SARS- CoV-2 virus genome allow specific amplification and detection of the viral RNA from all strains of SARS-CoV-2 from respiratory specimens. The Human RNase P gene is used as Internal control to monitor viral RNA extraction efficiency and assess amplifiable RNA in the samples to be tested.
Product Configuration
Kit Components
- One-step RT-qPCR master mix
- One set of primer/probes specific to the Orf1ab, N and E SARS-CoV-2 genomic regions and primers/probe for human RNase P gene
- A Positive Control (PC), Extraction Control (EC) and a No Template Control (NTC)
Pack Size
24-reaction per kit, 48-reaction per kit, 480-reaction per kit

*This product configuration image only shows the 24-reaction and 48-reaction pack size. 480-reaction pack size has 7 vials.
High Throughput & Ease of Use
High Throughput
93 samples (96-well plate) or 381 samples (384-well plate) per run
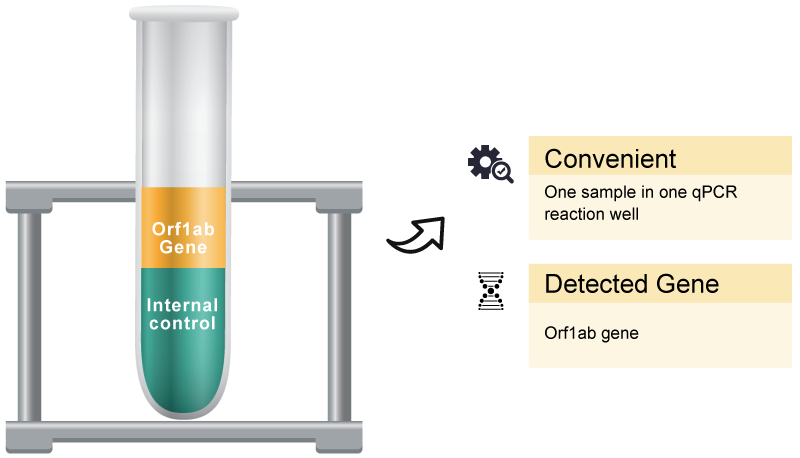
Detected Genes
Orf1ab
Ease of Use
One sample in one qPCR reaction well (1 assay mix/per sample)

Ultra-Sensitivity
Analytical Sensitivty
50 copies per mL of viral RNA
Ordering Information
US Customer Ordering Information (FDA EUA Authorized)
Product Name: QuantiVirus™ SARS-CoV-2 Multiplex Test Kit
24-Reaction Kit Catalog Number: DC-11-0017
48-Reaction Kit Catalog Number: DC-11-0018
480-Reaction Kit Catalog Number: DC-11-0019
The FDA EUA version and CE/IVD version have the same contents & configuration. Labelling is the only difference between the two versions.
Outside US Customer Ordering Information (CE/IVD Marked)
Product Name: QuantiVirus™ SARS-CoV-2 Multiplex Detection Test
24-Reaction Kit Catalog Number: DC-11-0015E
48-Reaction Kit Catalog Number: DC-11-0014E
480-Reaction Kit Catalog Number: DC-11-0016E
The FDA EUA version and CE/IVD version have the same contents & configuration. Labelling is the only difference between the two versions.
Resources
COVID-19 Total Solution
DiaCarta offers a COVID-19 total solution to support the fight against COVID-19, including the RT-PCR test kit, antibody IgG test kit, and CLIA lab service.

QuantiVirus™ SARS-CoV-2 Test Kit (RT-PCR Test - Detects 3 genes)

