Early Detection of the Vexas Syndrome
The test can find VEXAS patients missed by other tests due to low variant allele frequencies
Research Use Product
CLIA-Certified Lab Service
Research Service
Introducing QClamp® Plex VEXAS UBA1 Mution Test
DiaCarta has developed and fully validated a genetic test for VEXAS syndrome, called QClamp® Plex VEXAS UBA1 Mutation Test. The assay detects all the known mutations in the UBA1 gene associated with VEXAS syndrome. The assay uses DiaCarta’s proprietary QClamp® Plex technology to increase assay sensitivity and specificity.
Mutations in the UBA1 gene can be detected in the patient’s peripheral blood sample. There is no need to obtain bone marrow sample for the QClamp® Plex VEXAS UBA1 Mutation Test.
UBA1 mutation: DiaCarta has leveraged the proprietary XNA technology to increase the assay sensitivity so the somatic UBA1 mutations can be detected even at low frequencies
Identifying overlooked VEXAS patients: we have found other VEXAS patients missed by other tests due to low variant allele frequencies
Shorter turnaround time: unlike NGS, our multiplex VEXAS syndrome test has a shorter turn-around time, and no bioinformatic analysis is necessary.
VEXAS Syndrome
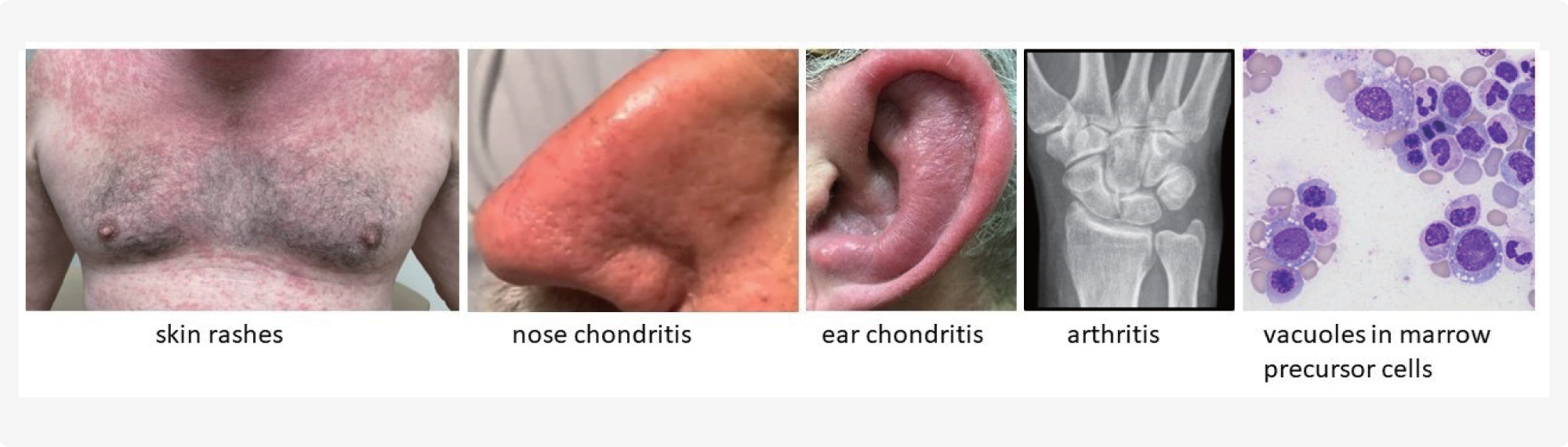
VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome is a chronic, progressive disease that causes severe systemic anti-inflammatory and hematological symptoms, including skin rashes that may be painful and swelling, and pain in nose and ears (nose and/or ear chondritis), cough and shortness of breath, pain and swelling in joints, inflammation of blood vessels, and failure of the bone marrow (e.g., macrocytic anemia, myelodysplastic syndrome). The disease affects multiple organs and is often misdiagnosed.
UBA1 Mutation
Fortunately, we now know the UBA1 gene somatic mutations have caused the disease. This allows early detection of VEXAS syndrome and improvement of the disease management.
Identifying Patients
Identifying patients with VEXAS syndrome is very challenging, and the diagnosis is often delayed or missed. Clinical vigilance and collaboration among clinicians (Rheumatology, Hematology, Dermatology, etc.) and pathologists are required to diagnose the disease without delay.
Genetic screening for pathogenic UBA1 variants should be considered in patients with vasculitis, autoinflammatory diseases or RP (Relapsed Polychondritis), especially male patients with skin lesions.
Patients with MDS (myelodysplastic syndromes) and AD (autoimmune disorders) who have characteristic vacuoles in myeloid and erythroid precursor cells should be screened for UBA1 mutation.
The DiaCarta offerings
PRODUCT
QClamp® Plex VEXAS Syndrome Test is a research product.
- Pack size: 10 samples
- Catalog number: DC-10-1003
SERVICE
QClamp® Plex VEXAS Syndrome Test is available at DiaCarta CLIA as a Lab-Developed-Test (LDT) or a research use test.
- Specimen(s) required: The preferred specimen is 3 mL whole blood in an EDTA (lavender-top) tube (minimum volume 1.0 mL). An alternative specimen is 2-3 mL bone marrow aspirate collected in an EDTA tube or extracted DNA sample from a CLIA-certified laboratory.
- Storage and transportation temperature: Room temperature or refrigerated.
- Specimen stability: Room temperature: 7 days; Refrigerated: 7 days; DO NOT freeze specimen.
- Turnaround time: 3 business days after receiving sample at DiaCarta CLIA-certified laboratory.
