RET Fusion Detection Test for Lung Cancer
XNA technology helps increase assay sensitivity and specificity
Research Use Product
Research Service
Early Detection/Screening
Diagnosis
Therapy Selection
Therapy Monitoring
Introducing QFusion™ Test
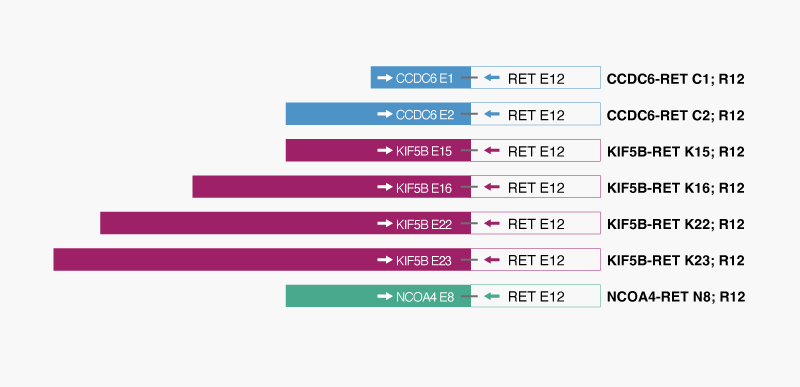
The QFusion™ RET Fusion Gene Detection kit is an XNA-based real-time RT-qPCR based in vitro diagnostic test intended for qualitative and indiscriminatory detection of seven RET fusions (two CCDC6-RET, four KIF5B-RET, and one NCOA4-RET fusions) in RNA extracted from cells, tissues, and formalin-fixed paraffin-embedded (FFPE) samples. The kit identifies the presence or absence of fusions but does not specify the exact fusion partner and truncation position of the fusion.
XNA-based RET fusion detection can increase assay sensitivity (reducing false negatives) and specificity (reducing false positives)
Analytical sensitivity: as low as 50 copies of fusions can be detected with 100%
Reproducibility: inter-reproducibility: 0.8 to 3.3% depending on the targets; intra-reproducibility: 0.7 to 3.3% depending on the targets
Validation of qPCR instruments: Bio-Rad CFX 384, Roche LightCycler 480II, and Thermo Fisher Scientific QuantStudio 5
Lung cancer is the most commonly occurring malignancy in both men and women worldwide. Genomic alterations, including structural rearrangements in lung cancer, have significant predictive value in treating disease.
Rearranged during transfection (RET) fusion in non-small cell lung cancer (NSCLC) accounts for 1–2% of cases. It is common in non-smoking lung adenocarcinoma (LUAD) patients. Among > 50 RET fusion variants that have been identified, KIF5B-RET and CCDC6-RET are the most common ones.
The RET-positive patients are generally younger than the average NSCLC patients and smoked little or never smoked. Fusions between the kinase domain of RET and the N-terminal region of other gene partners (RET fusion-positive) result in ligand-independent constitutive activation of RET, promoting cell proliferation and survival. Among the fusion gene partners, the most common partner is KIF5B (70-80% of cases), followed by CCDC6 and NCOA4. The QFusion ™ RET Fusion Detection Test detects 7 fusions shown on the image on the right side.
FDA approved two RET-specific tyrosine kinase inhibitors, selpercatinib and pralsetinib for the treatment of advanced RET-positive NSCLC. Therefore, the detection of any of these fusions is critical in therapy selection of these drugs.
Figure: Schematic of RET fusions detected by QFusion™ RET fusion Detection kit
XNA technology helps increase assay sensitivity and specificity
Powered by XNA technology, the QFusion™ RET Fusion Detection Kit has achieved a much higher analytical sensitivity than other commercial RT-qPCR kits and detection methods. XNA is a synthetic DNA analog in which the phosphodiester backbone has been replaced by a novel synthetic backbone chemistry. XNAs hybridize tightly to complementary DNA target sequences only if the sequence is a complete match. Binding of XNA to its target sequence blocks strand elongation by DNA polymerase.
