SARS-CoV-2 Variants Detection Test Kit
Screens for the SARS-CoV-2 virus and simultaneously identifies and differentiate all the new mutating COVID-19 variants.
Research Use Product
The QuantiVirus™ SARS-CoV-2 Variants Detection Test identifies seven important variants of SARS-CoV-2, including Alpha, Beta, Gamma, Delta, Delta Plus, Omicron, Epsilon, and Kappa. Using samples from sputum, saliva, and upper respiratory specimens, the test goes through RNA extraction and RT-qPCR steps to identify different variants on commonly used qPCR instruments. The test can also be adapted to high-throughput platforms if necessary. With a Limit of Detection of 100 copies/mL, the QuantiVirus™ SARS-CoV-2 Variants Detection Test has been validated using hundreds of clinical samples we have collected since January 2021.
Looking for a testing service? Our CLIA lab is also available for the COVID-19 testing! Learn more >
Product Highlights
- CE/IVD Marked
- Screen for infection of SARS-CoV-2 viruses in general
- Identifies and differentiate all the new mutating COVID-19 variants, especially variants of concerns (VoCs)
- Enhanced sensitivity (100 copies/mL) and specificity powered by DiaCarta’s proprietary XNA Technology
- Samples types: Sputum, saliva, nasopharyngeal, and oropharyngeal
- Compared to NGS, QuantiVirusTM SARS-CoV-2 Variants Detection Kit based on qPCR is faster, more reliable, and less expensive
Accurately Detects the Omicron Variant
Read our recent press release HERE
Validated Machines
Bio-Rad CFX384; Thermo Fisher (ABI) QuantStudio 5
COVID-19 Hotline
Email: support@diacarta.com
Phone #: +1 800-246-8878
GET A QUOTE NOW
Which Variants of Concerns (VoCs) are Targeted?
What is VoCs?
According to the WHO working definition, the variants of concerns (VoCs) refer to the virus variants with at least one of the three characteristics: (1) increase in transmission or worsening of the epidemiology; (2). Increase in virulence (3). Decrease in effectiveness of public health and availability of diagnostics and vaccines. WHO listed Alpha, Beta, Gamma, Delta, and Omicron as current VOCs.
VoCs and Significance of Detection
Accurately detecting different variants helps understand the infection transmission and severity of the health threat, and provides useful information for the variants spread in different regions and implementation of public health guidelines.
The Delta Variant
COVID-19 Variants of Concerns (VoC), especially the Delta variant, has significantly increased the infection rate and severity of the disease. With the increase of new round of infection by the Delta variant in many countries, the world is panicking again as vaccination does not stop the infection. Very quickly, the Delta variant has taken over the other variants and become the dominant variant in different countries.
The Omicron Variant
While the Delta variant is still dominating, a new variant named Omicron Variant has appeared first in Africa and is now detected in over 20 counties. The new variant recently classified as VOC by the WHO has also a fast transmission rate. Although some observations suggest that the new variants have milder symptoms in patients, it is too short to tell the potential danger to the human community.
List of Mutations Detected by QuantiVirus™ SARS-CoV-2 Variants Detection Kit
The QuantiVirus™ SARS-CoV-2 Variants Detection Kit is a real-time RT-PCR test intended for the qualitative detection of D614G, N501Y, T478K, L452R, K417N, and K417T mutations in the S gene of the SARS-CoV-2 virus. With different combinations of these mutations, we could detect all the major VOC.
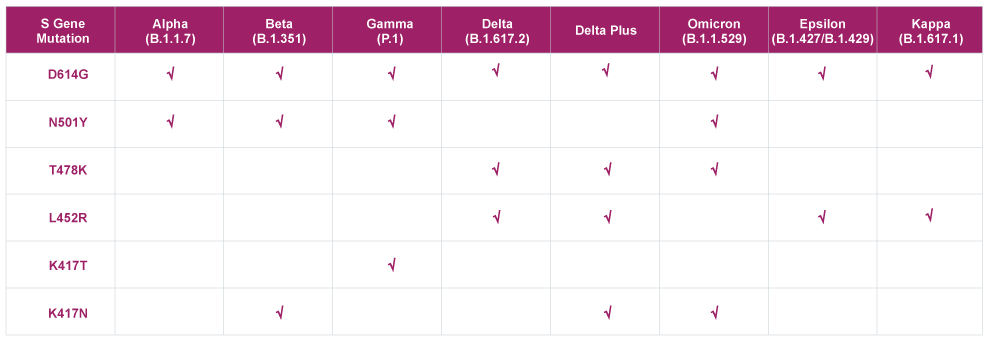
The Detection of the VoCs
Detection of the variant is more meaningful than just knowing the infection status. In addition, knowing which variant is circulated in a local area may help guide public policies in prevention and protection. For instance, detection of the Omicron variant particularly at this moment will tell
- How widespread is the new variant?
- Will this variant dominate and replace the Delta variant?
- How the symptoms or potential danger of this variant will be compared to the Delta variant?
- If there are any factors that one variant may become dominant in certain regions or populations but not in others?
Product Configuration
Kit Components
- Primer/Probe Mix
- XNA Mix
- One Step qRT-PCR Master Mix
- Positive Control, Negative Control, Extraction Control, and No Template Control
Pack Size
48-reaction per kit, 480-reaction per kit

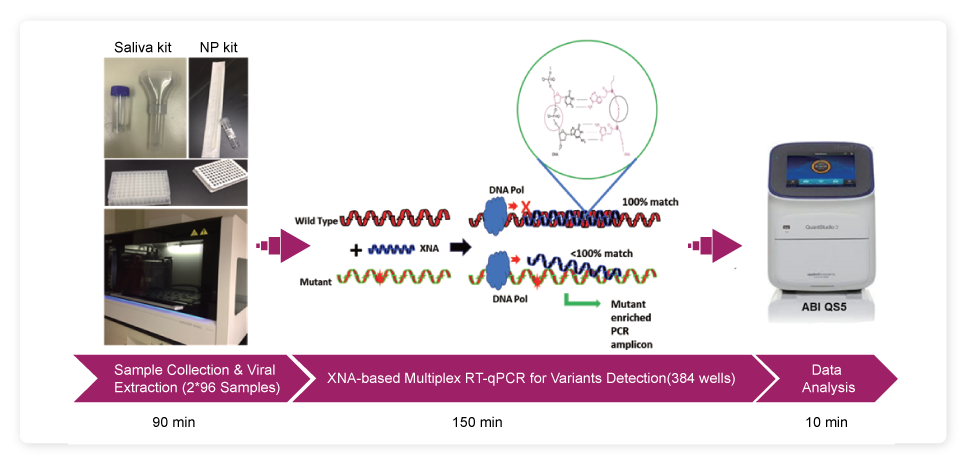
Workflow of QuantiVirus™ SARS-CoV-2 Variants Detection Kit
Sampling Methods
Sputum, saliva, and upper respiratory specimens taken using nasal or oropharyngeal swabs.
Viral RNA Extraction
Isolation of viral RNA with sample lysis or extraction to obtain qPCR templates. Can be adapted to high-throughput platform.
RT-qPCR
Kit performance is validated on three most popular qPCR instruments: ABI Quant-Studio 5 (Thermo), CFX384 (Bio-Rad), and ABI 7500 Fast Dx (Thermo).
XNA Technology
The QuantiVirus™ SARS-CoV-2 Variants Detection Kit is based on xenonucleic acid (XNA) mediated PCR clamping technology. Compared to other qPCR probe-based kits, the addition of an XNA, whose sequence is a complete match to the wild-type DNA, to a PCR reaction, blocks amplification of wild-type DNA allowing selective amplification of mutant DNA, assuring the specificity and avoiding false-positive results.
Why qPCR Genotyping, not Sanger or Whole Genome Sequencing?
Genotyping by qPCR has advantages for known variants compared to the sequencing technologies because it is fast and focuses on only a few mutations. Sequencing on the other hand is good for discovering new variants or confirming genotyping methods by qPCR if necessary. Sequencing, in general, takes much more time and is less cost-effective compared to genotyping by qPCR and when variants are known.
Who will Benefit from the QuantiVirus™ SARS-CoV-2 Variants Detection Kit?
Public health professionals who want to know the spreading of the Omicron variant compared to the presence of the Delta variant

Research clinical labs who want to study how the different mutations will affect the patients’ health

Pharmaceutical companies who are planning to develop the vaccine to the new Omicron variant may want to profile the vaccine recipients
Clinical Evaluation and Clinical Sample Testing
Clinical Evaluation
Clinical evaluation using the three qPCR instruments [ABI QuantStudio 5 (Thermo), CFX384 (Bio-Rad), and ABI 7500 Fast Dx (Thermo)] show 100%, 100% and 95% agreement with the spiked sputum sample at 1xLoD (1×100 copies/mL), and 100% agreement at all other concentrations including 200 copies/mL, 300 copies/mL, and 500 copies/mL. All the 30 negative samples were tested negative.
Clinical Sample Testing
Using the QuantiVirus™ SARS-CoV-2 Variants Detection Kit, we screened 374 positive samples and 102 negative samples collected in our own lab. For all the positive and negative samples testing for N501Y or D614G variant, the clinical sensitivity was 100.0% (95% CI: 98.7%-100.0%), specificity was 100% (95% CI: 95.5%-100.0%), PPV was 100.0% (95% CI: 97.4%-100.0%) and NPV was 100.0% (95% CI: 95.5%-100.0%).

Assay Performance
The assay performance was evaluated for intra- and inter-assay reproducibility, inter-instrument reproducibility, cross-reactivity with 21 known viruses.
Ordering Information
US Customer - Research Use Only Product
Product Name: QuantiVirus™ SARS-CoV-2 Variants Detection Kit
48-Reaction Kit Catalog Number: DC-11-0057R
480-Reaction Kit Catalog Number: DC-11-0058R
Outside US Customer: CE Marked Product
Product Name: QuantiVirus™ SARS-CoV-2 Variants Detection Kit
48-Reaction Kit Catalog Number: DC-11-0057
480-Reaction Kit Catalog Number: DC-11-0058
Resources
COVID-19 Total Solution
DiaCarta offers a COVID-19 total solution to support the fight against COVID-19, including the RT-PCR test kit, antibody IgG test kit, and CLIA lab service.

QuantiVirus™ SARS-CoV-2 Test Kit (RT-PCR Test - Detects 3 genes)

