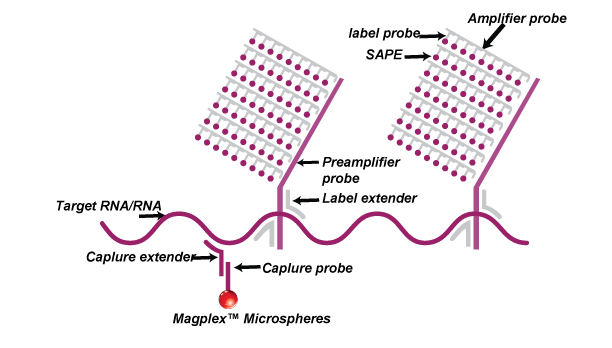
The Luminex-based platform uses capture probes that are conjugated to magnetic beads and the fluorescent signals generated from streptavidin-PE are detected on Luminex MAGPIX.
DiaCarta provides three products for QuantiDNATM assays:
QuantiDNA™ Direct cfDNA Test
Signal detection by Luminex MAGPIX
Catalog Number: DC-08-0096R
Pack Size: 96 Reactions.
Research Service is also available.
QuantiDNA™ Radiotherapy Toxicity Measurement Assay
Signal detection by Luminex MAGPIX
Catalog Number: DC-08-0296R
Pack Size: 96 Reactions.
Research Service is also available.
QuantiDNA™ Direct Human Residual Host Cell DNA Assay
Signal detection by Luminex MAGPIX
Catalog Number: DC-08-0396
Pack Size: 96 Reactions.
Research Service is also available.
GET A QUOTE NOW
QuantiDNA™ Assays are Based on SuperbDNA™ Technology
QuantiDNA™ assays are based on SuperbDNA™ technology that modifies the bDNA technology to improve assay sensitivity. The test uses a series of hybridization to capture the DNA molecules in the sample and amplify the chemical signal labeled on the specific probes rather than amplifying the targets themselves. The specific probe design allows accurate quantification of target DNA. Each oligonucleotide probe set contains two types of synthetic probes, capture extenders (CEs) and label extenders (LEs) that hybridize and span contiguous sequences of DNA. The 3′ tails of CEs bind to the DNA capture probes which have been conjugated to magnetic beads.
The LEs bind both to the target DNA and the Preamplifier Probe. One preamplifier probe has 20 binding sites for Amplifier Probe which possesses 20 binding sites for Label Probe. Therefore, one signal will be eventually amplified by 400 folds.
Finally, Biotinylated Label Probes will bind to Streptavidin phycoerythrin (SAPE) and the fluorescence signal on the beads can be detected by Luminex instruments such as MAGPIX®.
Comparison of the Two Platforms of Direct Human DNA Quantification Assays
Plate-based Platform
With Luminometer
The Plate-based platform captures probes that are pre-coated on the bottom of 96 well-microtiter plates and signals are detected using luminometers.

Capture Probe Carrier: Pre-coated on microtiter plates
Incubation Apparatus: Thermal incubator
Detection Instrument: Luminometer
VznHealth™ Applications
- VznHealth™ Direct cfDNA Test
- VznHealth™ Radiotherapy Toxicity Measurement Assay
- VznHealth™ Direct Human Residual Host Cell DNA Assay
Detection Instrument: Luminometer

QuantiReader™ Benchtop Luminometer
Plate-based Platform
With Luminex
The Beads-based platform captures probes are conjugated to magnetic beads and signal are detected on Luminex MAGPIX

Capture Probe Carrier: Conjugated to magnetic beads
Incubation Apparatus: Thermal shaker
Detection Instrument: Luminex MAGPIX
QuantiDNA™ Applications
- QuantiDNA™ Direct cfDNA Test
- QuantiDNA™ Radiotherapy Toxicity Measurement Assay
- QuantiDNA™ Direct Human Residual Host Cell DNA Assay
Detection Instrument: Luminex MAGPIX

VznHealth™ and QuantiDNA™ Assays Use Different Platforms for Testing but Give Similar Testing Results
Comparison of DNA Quantitation Results of
Plate-Based Assay and Beads-Based Assay
Assay Linearity and Performance
The performance comparison of the two DNA quantitation platforms is summarized in the table on the right side. Both platforms show the sensitivity that can measure low picogram of DNA in 20μl assay or very low ng/ml DNA. This range fits the healthy and cancer patient’s DNA detection with assay variation within 15%. In addition, no interference is found for the proteins or chemicals commonly found in plasma or reagents added to the plasma for DNA storage.
| VznHealth™ Direct cfDNA Test | QuantiDNA™ Direct cfDNA test | |
|---|---|---|
| Detection Limit | 0.39 ng/ml (7.8 pg in 20 µl assay) | 0.09 ng/ml (1.8 pg in 20 µl assay) |
| Quantitation Range | 0.39 to 50 ng/ml | 0.09 to 25 ng/ml |
| Intra-Assay Reproducibility | <15% | <10% |
| Inter-Assay Reproducibility | <15% | <15% |
| Interference from Hemoglobin | No | No |
| Interference from cholesterol | No | No |
| Interference from EDTA | No | No |
The cfDNA Test Result Comparison
The cfDNA test result comparison for a cancer patient before and after radiation therapy using the QuantiDNA™ assay (bead-based) and VznHealth™ assay (plate-based) can be found in the figure on the right. Both VznHealthTM assay (plate-based) and QuantiDNATM assay (bead-based) test are used for measurement of cfDNA concentration change for one prostate cancer patient after 1 to 5 days of radiation treatment versus cfDNA levels before treatment (in absolute number or expression as fold change).

