Radiation Therapy Toxicity Measurement (RadTox™)
A valuable aid to mitigate adverse events of radiation therapy and a liquid biopsy triage tool for pan-cancer radiation/chemotherapy
The Radiotherapy Toxicity Measurement Assay is developed to monitor tissue damage-related toxicity from radiation therapy. The measurement fulfils an unmet medical need in cancer radiation therapy by enabling the direct monitoring of side effect severity within days of radiation treatment initiation. The assay would allow dose escalation to tumors in less sensitive patients, aid development of radiotoxicity mitigators, and reduce overtreatment risk for sensitive patients. The radiotherapy toxicity assay provides fast, cost-effective quantitation of liquid biopsy cfDNA sample from cancer patients before and after radiation therapy and provides patient information for estimates of dosage usage and general radiation resistance.
QuantiDNA™ DNA Measurement Assay
Signal detection by a luminometer.
Catalog Number: DC-08-0092R (Research Use Only) and DC-08-0092E (CE/IVD Marked)
Pack Size: 96 Reactions.
LDT Service is also available.
QuantiDNA™ Direct cfDNA Test
Signal detection by Luminex MAGPIX
Catalog Number : DC-08-0096R (Research Use Only) and DC-08-0096E (CE/IVD Marked)
Pack Size: 96 Reactions.
LDT Service is also available.
Input DNA
10 µl plasma
RadTox™ LDT Test is Available
RadTox™ LDT (Laboratory Developed Test) Test developed at DiaCarta is used to evaluate patient radiotherapy toxicity at an early stage of therapy as a reference to physicians. Radiation is a common strategy for the treatment of cancer patients. However, people differ in sensitivity to radiation. Although a carefully picked radiation plan fits most people, a small fraction of people is either more sensitive or more tolerant to the radiation. The radiation treatment plan may cause unnecessary damage to the sensitive populations, but the tolerant population may not achieve optimal results.
Contact DiaCarta to order RadTox™ Test Service.
Call +1 800-246-8878
Email: order@diacarta.com
GET A QUOTE NOW
Advantages of the Radiation Therapy Toxicity Measurement Assay

Personalized Radiotherapy Toxicity Monitoring
The first liquid biopsy cfDNA in vitro diagnostics test for real-time assessment of low and high radiation toxicity

Significant Clinical Support
Real-time radiation dose monitoring helps aid doctors to evaluate and optimize radiation dosage and reduce patients’ risk for severe radiation toxicity
Streamlined Workflow
Direct cfDNA measurement without DNA/RNA extraction or cleaning. No PCR or target DNA amplification

Minimal Sample Requirement
Only 10 µl plasma needed
Drawbacks on the Current Gold Standard for Radiation Therapy Tissue Damage Estimation
Radiation therapy to treat cancer patients is widely used worldwide, although doctors and patients recognize potential serious treatment side effects, either with radiation therapy alone or in combination with approved agents or drugs. The current gold standard for clinical evaluation of tissue damage caused by radiation therapy:
Is based on population statistics, not personalized
Relies on the dose volume histogram (DVH) or maximum tolerated dose (MTD) studies of combination of radiation and the drug
Radiotherapy dose is commonly set to achieve remissions or cures in patients without concurrently causing grade >3 toxicity level in over 5 to 10% of patients, based on population statistics correlated with daily dose and dose distributions. However, this standard dose regimen restricts the high-dose tolerating patients from getting curative benefit from radiotherapy and fails to protect the 5% highly sensitive patients with reduced radiation dosage or even no radiation therapy. Some highly sensitive patients may have pre-existing conditions that could increase tissue or organ damage and result in additional medical complications. Low dosage of radiation such as 20 Gy may even cause patient death in some cases.
An Optimal Radiation Biomarker is Necessary
An effective biomarker for radiation therapy safety/toxicity measurement can help
Reduce risk and cost of life-threatening toxicity
Avoid unnecessary treatment
Lower healthcare costs
The ideal biomarker for radiation toxicity monitoring should have the following characteristics:
Early detection: determine the personalized tolerance of an individual patient’s normal and tumor tissues to radiation therapy
Quantitative: provides estimate for low, medium or high risk
Quick and Easy: is performed during a course of treatment to adjust treatments as needed
Radiation treatment is required by 70 percent of cancer patients. However, despite decades of advances in cancer care and technology, there remain many fundamental challenges affecting safe and efficacious delivery of radiation treatment. Currently, there is no reliable method for determining treatment risk caused by radiotherapy.
In the past decades, great progress has been made in the development of imaging instruments, but little progress is shown in the development of biological and chemical testing tools. Although new biological or chemical agents are being approved for clinical use, not many of them have been tested for use with a combination of these agents and radiation therapy. Currently, there is no clinical method for determining treatment risk caused by radiation toxicity and for personalizing the radiation dose during the course of treatment. The most closely related technology is non-personalized biodosimetry (TBI), such as lymphocyte counts, chromosomal aberrations, radical traps, cytokine profiles, and H2AX measurements. Development of new biomarkers is necessary for cancer patients and their doctors to understand radiation risks and to determine appropriate dosages.
A Promising Biomarker: cfDNA quantification
We discovered that plasma concentration of circulating cell-free DNA (cfDNA) concentration correlates with radiation doses, and further developed an assay to predict the level of radiation damage to cells by measuring cfDNA. The cfDNA level is proportionally correlated with circulating tumor DNA (ctDNA) shed from tumor cells (US patent #8,404,444) and therefore is used in our assay.
Most cancer patients have a similar baseline level of cfDNA in the blood. Radiation increases cfDNA to different levels for different individuals. By determining the cfDNA level in cancer patients’ blood after radiation, we can identify patients’ personalized radiation sensitivity and estimate the highest risk for radiation toxicity. Currently, there is no clinical method to determine treatment-induced toxicity. Compared with other technologies that estimate cell death or DNA damage, the DiaCarta Radiation Therapy Toxicity Measurement Assay provides early detection and is cost-effective and easy to use.
| Tumor Type | Patient Number | Average of cfDNA Content (ng/ml) |
|---|---|---|
| Colorectal Cancer | 135 | 26.43 |
| Malignant Breast Tumor | 202 | 26.16 |
| Gastric Malignant Tumor | 151 | 21.94 |
| Malignant Tumor of Esophagus | 167 | 19.75 |
| Non-Small-Cell Lung Carcinoma | 219 | 22.87 |
| Total | 874 | / |
| Competing Technology (Mechanism) | Specimen Type | Specimen Preparation | Analysis | Ease of Deployment/Affordability | Technical Feasibility | Regulatory Status |
|---|---|---|---|---|---|---|
| DiaCarta Method (Holistic Measure of Cell Death) | Plasma, Strech Tube, Finger Stick | None (Plasma Isolation) | Quantitative and Reliable | High | Simple Implementation and Interpretation | CE Marked Europe |
| Cytokines Arrays (Inflammation) | Plasma, Strech Tube, | Ice, Platelet Poor Spin, Immediate Freezing | Very few Reproducible or Quantitative ELISA Antibodies Exist | Medium | Difficult QC and Reproducibility | None |
| Biomics Array (Acute Response Pathways) | RNA Preservation Medium | DNA/RNA Purification and PCR | Proprietary Interpretation (Expected to be Race/Gender Specific) | Low | Proprietary Technology Required | None |
| DNA Repair (e.g. H2AX) | Biopsy Irradiated Cells Required | DNA/RNA Purification and Microscopy | Extreme Dependence on Time of Specimen Acquisition | Very Low | Proprietary Technology Required | None |
| DxTdrity (Mechanism Unknown) | Leukocytes from Whole Blood | DNA/RNA Purification and PCR | Proprietary Interpretation (Data Mining) | Medium (Shipping for Off-Site Analysis) | Proprietary Technology Required | REDI-Dx (CE Marked U.S.) |
The Radiotherapy Toxicity Measurement Assay is Powered by SuperbDNA™ Technology
The Radiation Therapy Toxicity Measurement Assay is a SuperbDNA™ technology-based quantitative assay using a luminometer or Luminex MAGPIX instrumentation. The test kits have the potential to scale up or be adapted to automation for high throughput operation by simply integrating into the workflow of radiation therapy centers.
Branched DNA (bDNA) technology is a widely used clinical platform to quantitatively detect specific nucleic acid sequences directly from the source without DNA/RNA purification or RT-PCR. bDNA quantitative hybridization technology has a wide dynamic range and is sensitive enough for applications intended to reliably detect very few target molecules. bDNA technology has several advantages over PCR technology and is commonly used for applications requiring a high degree of specificity and sensitivity.
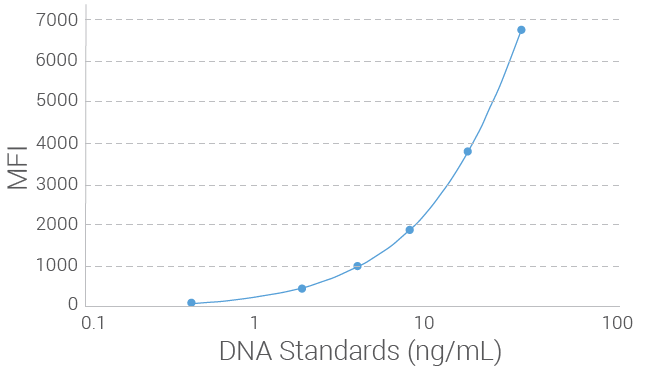
Limit of Detection (LOD) for Radiation Therapy Toxicity Measurement Assay
The limit of detection (LOD) of the Radiation Therapy Toxicity Measurement Assay was determined by measuring serially diluted genomic DNA at a
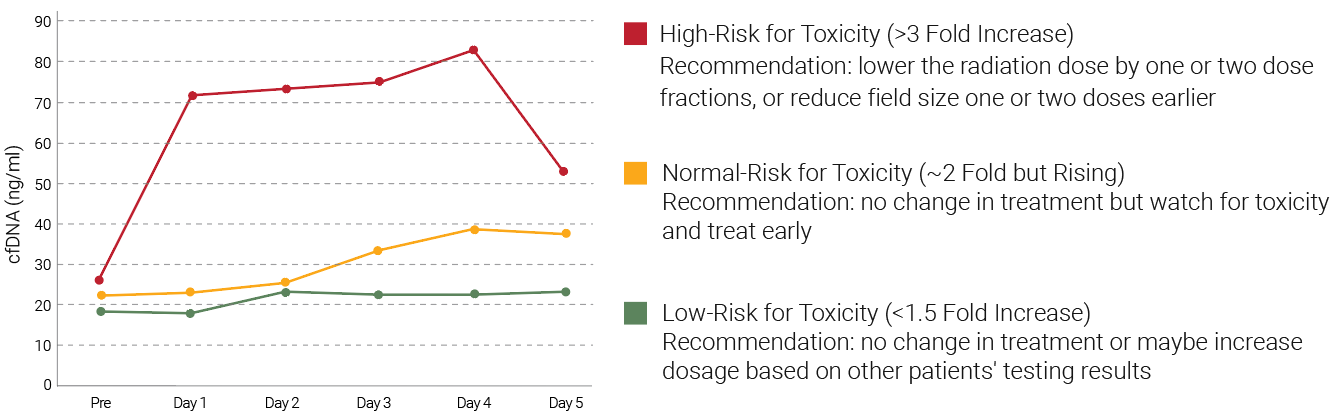
Clinical Evaluation of Patient Radiation Therapy Using Radiation Therapy Toxicity Measurement Assay
Among the 54 prostate cancer patients who received radiation therapy in a clinical study, <5% had a large increase in cfDNA. The patients could be divided into three groups: the low risk, normal and high-risk groups, and radiation therapy dosage suggestions can be given based on the risks determined by the cfDNA changes 24 hours after radiation therapy. The dataset on the right side shows the correlation of plasma cfDNA concentration with radiation dosage. The biomarker is an indicator for cfDNA before and after radiation therapy.
Webinar
Title: Creating the Optimal Liquid Biopsy: Post-Radiation Tumor Toxicity Measurement & A Highly Sensitive Early Colorectal Cancer Detection Test
Speaker: Paul Okunieff, M.D. (Professor and Chair, Department of Radiation Oncology, Gainesville and University of Florida Health Proton) and Jinwei Du,
Highlight:
- Define optimal liquid biopsy and its potential to achieve its promise in personalized medicine
- Current and proposed implementation of DiaCarta’s SuperbDNA™ and QClamp® technologies
- DiaCarta Radiation Therapy Toxicity Measurement Assay – Implementation & measurement of
tumor response and normal tissue toxicity after radiation - ColoScape™ – Discuss the future use of early identification of subclinical disease, cancer screening & early measurement of chemotherapy response
Resources
View recent press releases for the DiaCarta Radiation Therapy Toxicity Measurement Assay: