JAK2 Mutation Detection Tests
Aids in identifying cancer patients eligible for treatment and monitors response to therapy which can lead to improved outcomes in cancer patients.
CE/IVD Marked Product
Research Use Product
Research Service
Early Detection/Screening
Diagnosis
Therapy Selection
Therapy Monitoring
Introducing QClamp® JAK2 Test
The QClamp® JAK2 Mutation Detection Test is an in vitro diagnostic real-time quantitative PCR assay. Powered by XNA technology, the kit has achieved a much higher analytical sensitivity than some other commercial qPCR kits and other cancer gene mutation detection methods. QClamp® JAK2 Mutation Detection Test can reliably detect below 0.5% mutant DNA out of wild-type DNA for targeted mutations using only 10 ng DNA, providing data when the sample is scarce.
Detected codon: V617F mutation
Require only 10 ng of DNA input
Reliably detects below 0.5% VAF mutant DNA out of wild-type DNA for targeted mutations
Validated instruments: Roche LightCycler® 480II, Bio-Rad CFX384™, Bio-Rad CFX96™, and Thermo Fisher (ABI) QuantStudio™ 5
JAK2 Mutation and Cancer
JAK2 Introduction
Janus kinase 2 (JAK2) is a non-receptor tyrosine kinase that associates with the cytoplasmic domains of multiple cytokine receptors to activate downstream targets, including STATs and JAK2 itself.
JAK2 Mutations
V617F mutation in JAK2 leads to constitutive tyrosine phosphorylation activity and constitutive activation of STAT5. This mutation is common in the following cases: >90% of patients with polycythemia vera, 50% of patients with primary myelofibrosis and 60% of patients with essential thromobocytopenia.
Supporting Data
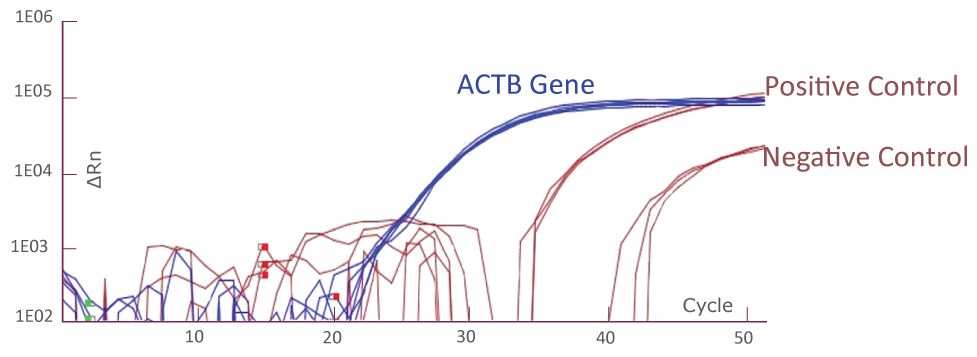
QClamp® JAK2 Mutation Detection Test amplification plot from ABI QuantStudio 5
Amplification plot showing triplicates of positive controls (mutant template and wild-type template mix) and negative controls (wild-type templates only) for JAK2 TaqMan amplification using ABI QuantStudio 5. Reference gene ACTB amplification from the positive and negative controls (total 6) is shown as well.
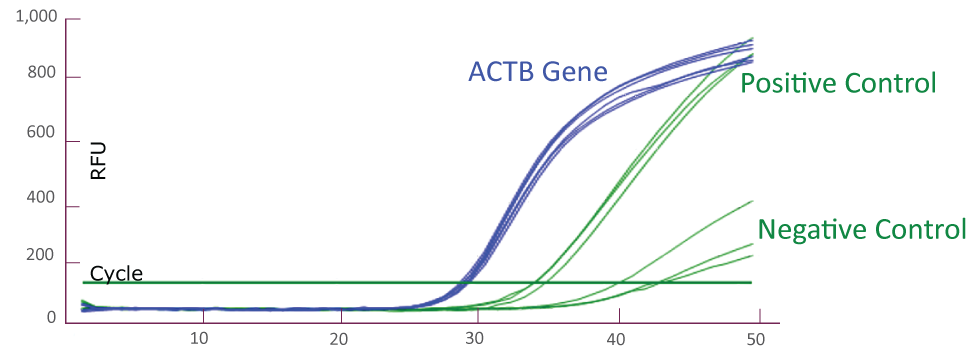
QClamp® JAK2 Mutation Detection Test amplification plot from Bio-Rad CFX384
Amplification plot showing triplicates of positive controls (mutant template and wild-type template mix) and negative controls (wild-type templates only) for JAK2 TaqMan amplification using Bio-Rad CFX384. Reference gene ACTB amplification from the positive and negative controls (total 6) is shown as well.
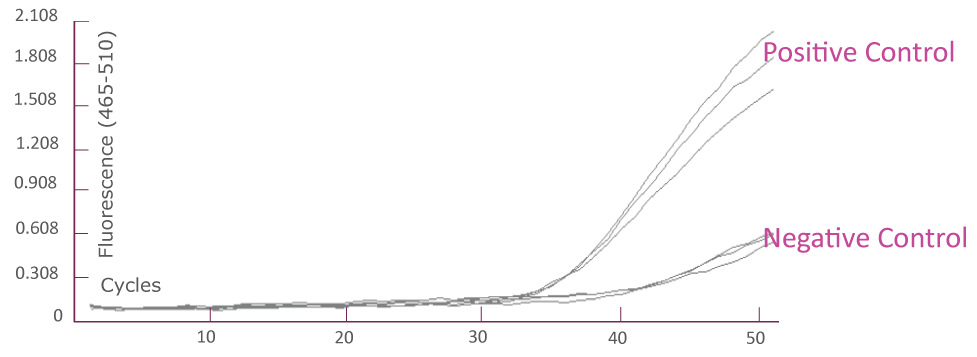
QClamp® JAK2 Mutation Detection Test amplification plot from Roche LightCycler® 480 (FAM)
Amplification plot showing triplicates of positive controls (mutant template and wild-type template mix) and negative controls (wild-type templates only) for JAK2 TaqMan amplification using Roche LightCycler® 480.
Streamlined Workflow (less than 4 hours assay run time)

Step 1: DNA Isolation & Quantification
Extract DNA from FFPE or plasma using a commercial DNA extraction kit and measure the concentration using fluorometric analysis

Step 2: set up qpcr
Mix the assay reagents, load into PCR plate, add controls and extracted DNA ~ 30-60 minutes
Step 3: Amplification parameters
Enter amplification parameters on
qPCR instrument, load PCR plate
and start the run ~ 2.5 hours
Step 4: Data analysis
Determine the presence or absence
of mutations according to the Cq
value cutoffs ~ 15 minutes
Product Specification
Catalog Number
Pack size: 10 samples – DC-10-1037 (CE/IVD version); DC-10-1037R (research use version)
Pack size: 30 samples – DC-10-0166 (CE/IVD version): DC-10-0166R (research use version)
Detected Codons
Codon 617
Kit Components
Primer/probe mix, XNA, master mix, positive control, negative control, non-template control
Input DNA
5-10ng/Reaction
Validated Instruments
Roche LightCycler® 480II, Bio-Rad CFX384™, Bio-Rad CFX96™, and Thermo Fisher (ABI) QuantStudio™ 5
Detection Channel
FAM; HEX
Detection Chemistry
TaqMan
Turnaround Time
Less than 4 hours
Stability
Stable for 12 months at -25 ℃ to -15 ℃
Most frequent JAK2 mutations detected by QClamp® JAK2 Mutation Detection Test
| Exon | Amino Acid Change | Nucleotide Change | Cosmic No. |
|---|---|---|---|
| 14 | V617>F | 1849G>T | 12600 |
Resources
Intended Use: QClamp® JAK2 Mutation Detection Test is CE/IVD-certified. This product is available outside the USA for diagnostic use (CE/IVD) and research use (RUO). In the USA, this product is provided for research use only (RUO) and not for diagnostic use. DiaCarta ships the CE/IVD version to locations outside the USA and sends the RUO version within the USA. Please call 1-800-246-8878 or email order@diacarta.com if you have questions or specific needs.
Shipping Condition: QClamp® JAK2 Mutation Detection Test will be shipped with dry ice. For domestic shipment, DiaCarta provides overnight delivery through FedEx Domestic Overnight Shipping Service. For international shipment, DiaCarta provides 3-7 days in transit through FedEx International Priority Shipping Service. Please contact DiaCarta if you prefer to use your shipping carrier.
