Companion Diagnostics
A range of customizable testing services powered by XNA technology
DiaCarta offers a range of customizable testing services using XNA technology
We are committed to providing companion diagnostics development services customized for your needs. In addition, we offer unique project management services to ensure your project is completed in a timely manner and is ready for regulatory submission.
We provide customized molecular tests on FFPE samples, liquid biopsy samples such as blood, and challenging small biopsies and cytology samples (fine needle aspirates, effusions, and CSF).
What is Companion Diagnostics and Why it is Important?
A therapeutic drug can have different results between patients with the same disease due to disease heterogeneity. Such heterogeneity can include, but is not limited to, different driver mutations in oncogenes of cancer patients. Patients may develop resistance to therapeutic drugs because of certain mutations in cancer genes and associated functional changes in the proteins that are targeted by the drugs. It is necessary to screen the patients for these particular cancer gene mutations so different therapeutic drugs can be used to target those mutations.
Companion diagnostics uses predictive biomarkers to match the most appropriate drug to the cancer patient and thus enhances treatment outcomes.
Companion Diagnostics Development Steps
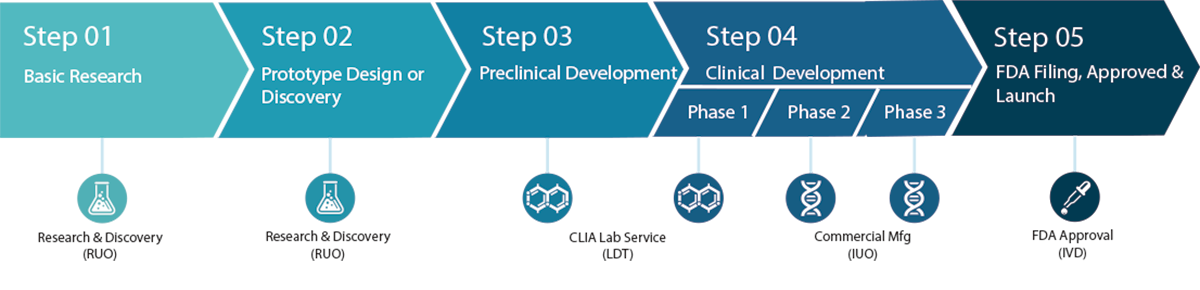
Although the concept of companion diagnostics has been around for 40 years and the steps for development of such tests are known, the progress has been slow. From 1998 to 2016, 167 cancer drugs have been approved by the U.S. FDA. However, only 10% of the treatments are used in companion diagnostics.
Alignment of the Drug and Diagnostic Development
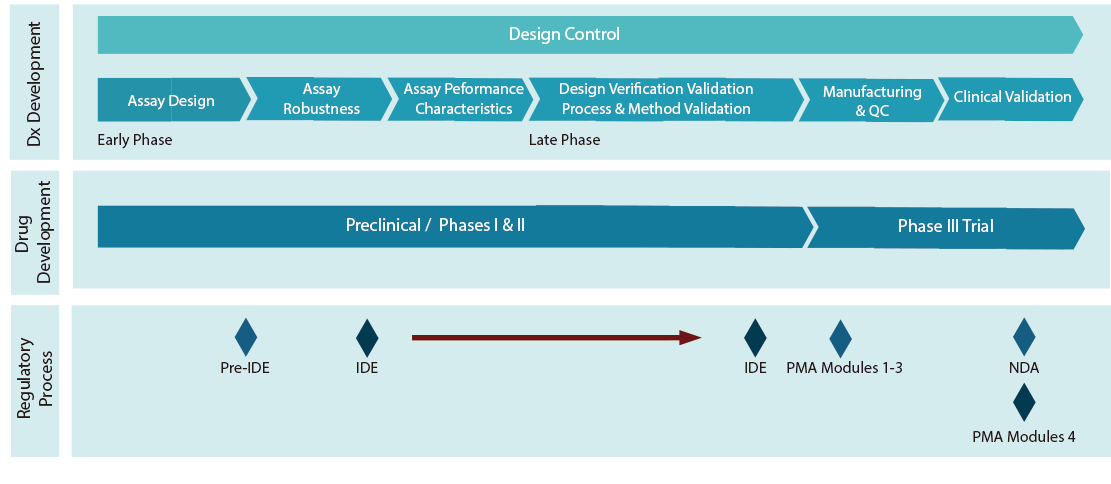
The majority of the approved companion diagnostics tests focus on cancer therapy. Using the knowledge of cell signaling pathway, companion diagnostics help predict patient treatment outcomes based on a tumor’s genetic make-up. With a better understanding of the mechanism of cancer development and the increased sensitivity of molecular diagnostics, development of companion diagnostics for different therapeutics drugs is rapidly underway. The market for the global diagnostic market is estimated to reach $6.51B from $2.17B in 2016, with a Compound Annual Growth Rate (CAGR) of 20.1% according to MarketsandMarkets.
The Challenge of Companion Diagnostics
ne key challenge for developing effective companion diagnostics is to coordinate drug development and companion diagnostics development so they can be aligned from both product and regulatory point of view.
Activating Mutations and Targeted Cancer Therapies
Currently, targeted therapies attack the following targets
Receptor tyrosine kinases such as EGFR, ERBB2, c-KIT and others
Non-receptor tyrosine kinases such as ABL and JAK2
Serine-threonine-lipid kinases, such as BRAF, PI 3 kinase, and mTOR
DNA damage and repair proteins, such as BRCA1 and BRCA2
Targeted Therapy for the EGFR Gene as an Example for Companion Diagnostics
One of the examples for companion diagnostics for lung cancer treatment is targeted EGFR therapy. Among the metastatic Non-Small Cell Lung Cancer (NSCLC) patients, about 30 to 50 % of Asian and 7 to 23% Western population has EGFR-activating mutations, such as exon 19 deletion or L858R. These patients are highly responsive to EGFR inhibitors, such as gefitinib and erlotinib. There is strong clinical evidence to support that these EGFR inhibitors only work with patients harboring the above activating EGFR mutants. However, almost 63% of the patients develop resistance after 8 to 14 months of drug treatment due to development of a new mutation in EGFR, T790M. Correct and in-time identification of EGFR mutations and using appropriate companion diagnostics is critical for the development of effective patient therapy strategies.
