RT-PCR Test with Nasopharyngeal or Oropharyngeal Swab

Standard Test is FREE (Covered by Insurance, Results in 24-48 Hours)
Drive-Through Testing with No Appointment Required

Same-Day Result Available at $150 (Test before 12PM)
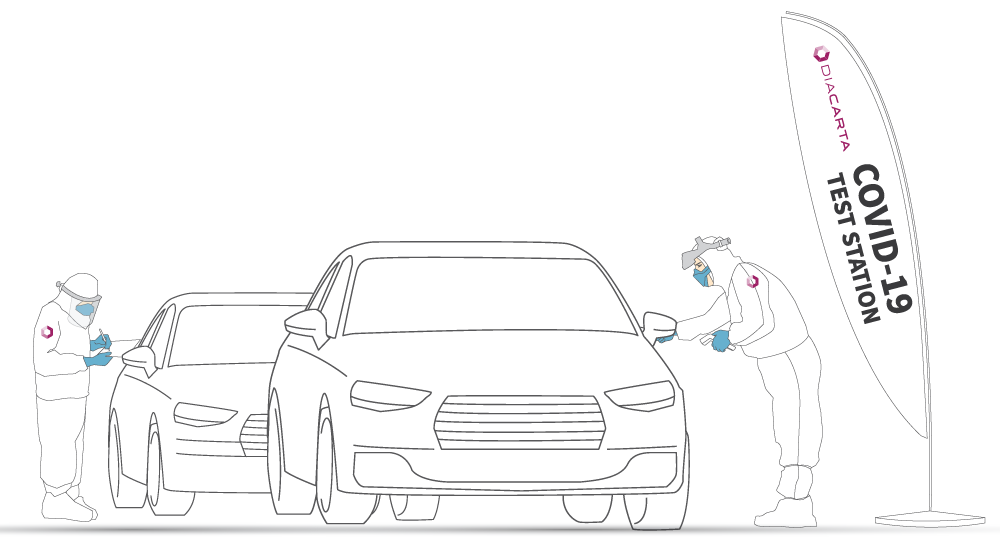
How does it Works?

STEP 1
Register at DiaCarta Clinical Laboratory
Follow the instruction below for registration. Print out your order form and make sure you have the order confirmation QR code on your order form.
Questions? Email support@diacarta.com or call 800-246-8878
Option 1: Standard Test (FREE, Covered by Insurance)
- Results in 24-48 hours
- No appointment needed
- Read step-by-step registration instruction
Option 2: Same-Day Result (Test before 12PM)
- Results in same-day (must complete testing by 12PM)
- Cost is $150, not covered by insurance
- Contact support@diacarta.com to book your appointment
STEP 2
Visit DiaCarta Clinical Laboratory
We are open 9 AM -5 PM Monday through Friday.
Lab address
Must Bring
- ID
- Order form with the QR code. Printed form is preferred. Or save the order form and QR code on your phone.
STEP 3
View your results on our secure web portal.
Standard Test: Your test report will be sent to the email address you provided during registration within 24-48 hours after lab receipt of your sample.
Same-Day Result: Your test report will be sent to the email address you provided during registration on the same day of your test. Note: same-day result only works if test before 12 PM.
You can also access your lab results by logging into our secure Patient Portal at https://lims.diacarta.com/patientportal/.


