QClamp® BRAF Mutation Detection Tests
Improved Sensitivity for Single Gene Mutation Detection
TThe QClamp® BRAF Mutation Detection Test for plasma and Formalin-Fixed Paraffin-Embedded (FFPE) samples aids in the identification of patients eligible for cancer treatment and in monitoring response to therapy, which can lead to improved outcomes in cancer patients. The QClamp® BRAF Mutation Detection Test is an in vitro diagnostic real-time qualitative PCR assay for the detection of somatic mutations in and near codon 600 on exon 15, in the BRAF serine/threonine-protein kinase gene, using purified DNA extracted from FFPE or plasma.
Powered by XNA technology, the QClamp® BRAF Mutation Detection Test has achieved a much higher analytical sensitivity compared to other commercial qPCR kits and other cancer gene mutation detection methods. QClamp® BRAF Mutation Detection Test is able to detect reliably 0.1% to 0.5% mutant DNA out of wild-type DNA for targeted mutations, providing lower detection limit compared to similar assays available in the market due to robust enrichment of mutant sequences while suppressing amplification of wild-type sequences.
Product Catalog
QClamp® BRAF Mutation Detection Test – CE Version: DC-10-0197
QClamp® BRAF Mutation Detection Test – Research-Use Version: DC-10-0197R
Pack Size: 30 Samples
Detected Codon
Codon 600
Service Offering
We provide research service for QClamp® BRAF Mutation Detection Test.
GET A QUOTE NOW
Advantages of QClamp® BRAF Mutation Detection Test

ULTRA-SENSITIVE
Reliably detects 0.1% to 0.5% VAF mutant DNA out of wild-type DNA for targeted mutations

SAMPLE READY
Suitable for plasma and FFPE samples

LOW INPUT DNA
Minimum 5ng input DNA per reaction. Less than 2 tubes of blood (10mL each) needed for cfDNA

COMPREHENSIVE COVERAGE
Covering all relevant somatic mutations of BRAF oncogene
FAST RESULTS
Less than 4 hours of assay run time

GREAT VERSATILITY
Validated on the most common qPCR machines with minimized variability
BRAF Mutation and Cancer
BRAF Introduction
The B-type Raf Kinase (BRAF) protein is a serine/threonine kinase that has important roles in regulating the MAP kinase/ERK signaling pathways, affecting cellular proliferation, differentiation, and programmed cell death. A BRAF mutation is commonly found in many human cancers including malignant melanoma, colorectal cancer, lung cancer, papillary thyroid carcinoma, non-small cell lung carcinoma, and adenocarcinoma of the lung.
BRAF Mutations
The most common mutations in BRAF occur in codon 600, where an amino acid substitution in the activation segment of the kinase domain creates a constitutively active form of the protein. The V600E and V600K mutations are found in high frequencies in human cancer at 70-90% and 10-15%, respectively. BRAF mutations are generally found in tumors that are wild-type for KRAS, NRAS, and EGFR. Therefore, BRAF c600 mutation may serve as a biomarker for diagnosis, prognosis, and treatment options for cancer patients.
BRAF V600E and Targeted Cancer Therapy
Drugs, such as RAF inhibitor vemurafenib inhibit the ERK pathway and cell proliferation and lead to 80% response rate in metastatic melanoma patients carrying the BRAF V600E mutation. However, these drugs do not have therapeutic effects for colorectal cancer patients carrying the same mutation. This is due to overexpression of EGFR in BRAF V600E colorectal cancer cells, but not in melanoma cells because they overcome the inhibition of the BRAF mutant kinase activity. In these colorectal cancer patients, use of tyrosine kinase inhibitors such as gefitinib, erlotinib, and cetuximab together with vemurafenib has a synergistic effect.
Supporting Data for QClamp® BRAF Mutation Detection Test
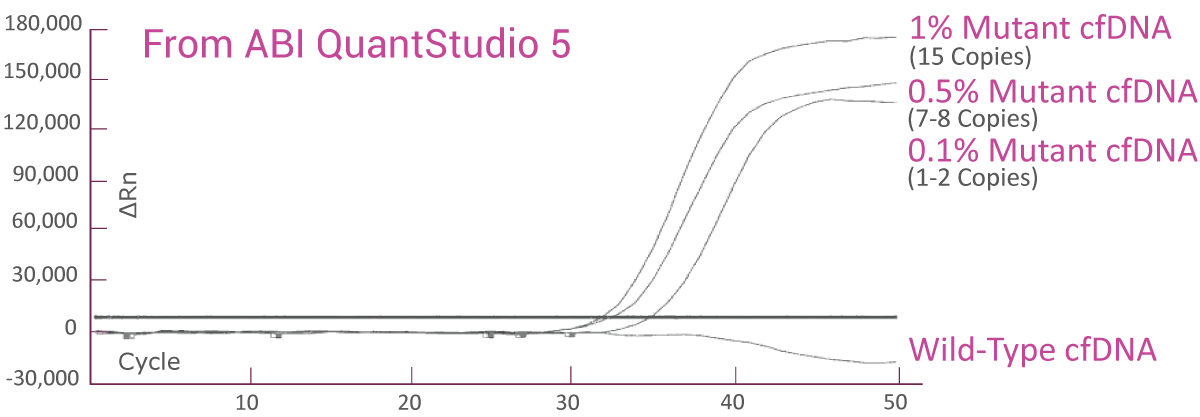
QClamp® BRAF Mutation Detection Test analytical sensitivity in plasma: detects as low as 0.1% VAF mutant DNA in a 5ng input
cfDNA reference material from SeraCare with known BRAF V600E VAF was diluted to 1%, 0.5% and 0.1% VAF with 2.5ng/μl of wild-type cfDNA. The BRAF V600E assay was able to detect as low as 1-2 copies of BRAF V600E mutant (0.1% VAF) in the background of wild-type cfDNA.
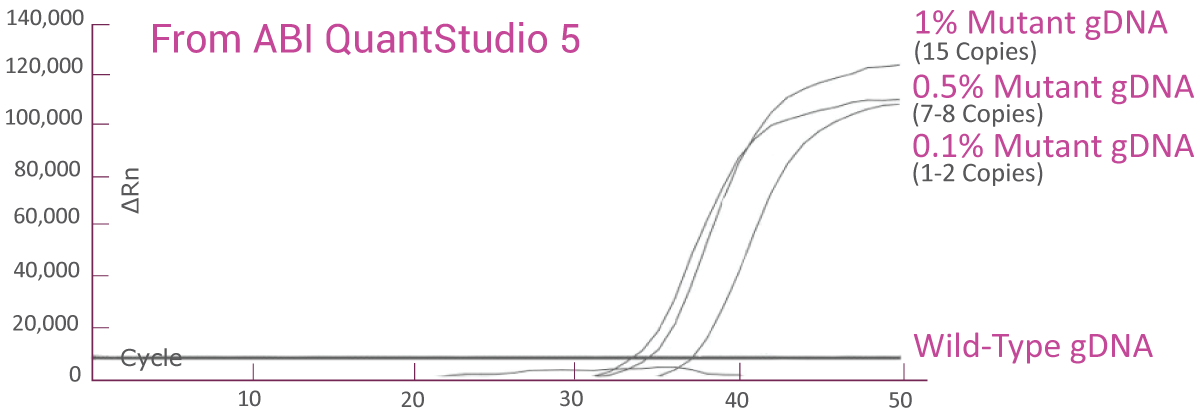
QClamp® BRAF Mutation Detection Test analytical sensitivity in FFPE: detects as low as 0.1% VAF mutant DNA in a 5ng input.
Genomic DNA (gDNA) reference material from Horizon Discovery with known BRAF V600E VAF was diluted to 1%, 0.5% and 0.1% VAF with 5 ng/μl wild-type cfDNA. The BRAF V600E assay was able to detect as low as 3-4 BRAF V600E mutant copies (0.1% VAF) in the background of wild-type gDNA.
Streamlined Workflow for QClamp® Gene Mutation Detection Tests

Step 1: DNA Isolation & Quantification
Extract DNA from FFPE or plasma using a commercial DNA extraction kit and measure the concentration using fluorometric analysis

Step 2: set up qpcr
Mix the assay reagents, load into PCR plate, add controls and extracted DNA ~ 30-60 minutes
Step 3: Amplification parameters
Enter amplification parameters on
qPCR instrument, load PCR plate
and start the run ~ 2.5 hours
Step 4: Data analysis
Determine the presence or absence
of mutations according to the Cq
value cutoffs ~ 15 minutes
Resources
Catalog Number
CE Catalog #: DC-10-0197
Research-use-only (RUO) catalog #: DC-10-0197R
Pack Size
30 samples
Detected Codons
Codon 600
Sample Type
Plasma and FFPE
Input DNA
5-10ng/Reaction
Validated Instruments
Roche LightCycler® 480, Bio-Rad CFX384 and ABI QuantStudio 5
Detection Channel
FAM; HEX
Detection Chemistry
TaqMan
Turnaround Time
Less than 4 hours
Stability
Stable for 12 months at -25 ℃ to -15 ℃
Most frequent BRAF mutations detected by QClamp® BRAF Mutation Detection Test
| Exon | Amino Acid Change | Cosmic No. |
|---|---|---|
| 15 | p.V600E | COSM476, COSM475, COSM1131 |
| p.V600K | COSM473, COSM26487 | |
| p.V600R | COSM474, COSM21617 | |
| p.V600D | COSM477, COSM36128 |
Download
Ordering Information
For products that are in stock, DiaCarta will arrange shipment in 1-3 days. For products that are on backorder, DiaCarta will arrange shipment in 3-5 weeks.
Intended Use: QClamp® BRAF Mutation Detection Test is CE/IVD-certified. Outside
Shipping Condition: QClamp® BRAF Mutation Detection Test will be shipped with dry ice. For domestic shipment, DiaCarta provides overnight delivery through FedEx Domestic Overnight Shipping Service. For international shipment, DiaCarta provides 3-7 days in transit through FedEx International Priority Shipping Service. Please contact DiaCarta if you prefer to use your own shipping carrier.
